This page provides you with an overview of the cleanroom classes according to the Good Manufacturing Practice (GMP) guidelines and the ISO 14644-1 standard, which is used worldwide to classify cleanrooms.
Cleanroom classes according to GMP
According to Good Manufacturing Practices (GMP), there are various cleanroom classes that define the cleanroom requirements for pharmaceutical and medical facilities. The cleanroom classes are defined based on the number of particles per cubic metre of air. Here are the most important classes:
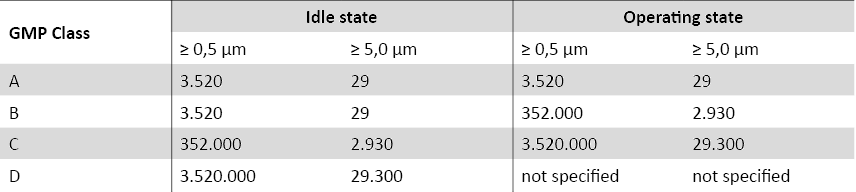
Annex 1 to the EC GMP Guidelines (status 2008) Subject to errors
Cleanroom classes according to ISO 14644-1
The cleanroom classes according to ISO 14644-1 are internationally standardised and define the requirements for air purity in cleanrooms. The ISO 14644-1 standard defines the number of particles per cubic metre of air for different cleanroom classes.
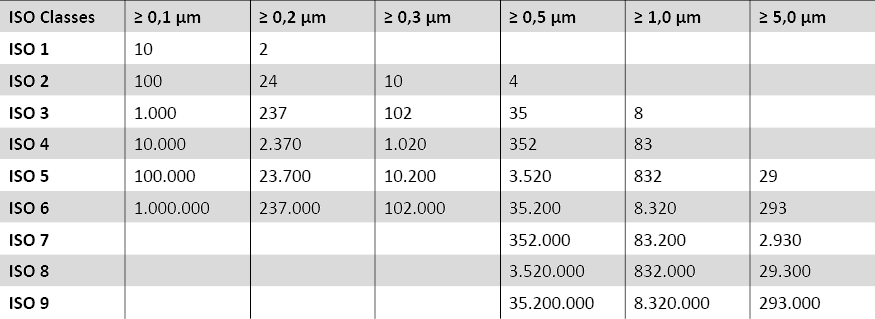
DIN EN ISO 14644-1 Subject to errors
Summary
Both classification systems are used to define cleanliness and cleanroom conditions in various industrial sectors. The choice of cleanroom class depends on the requirements of the specific application, with GMP classes often used in pharmaceutical production and ISO classes in various industrial and technical applications. The exact choice of cleanroom class depends on the standards and guidelines that apply to the specific industry.
Kirsch cleanroom refrigerators set the standard for quality and performance in cleanroom applications by meeting the stringent requirements of cleanroom class GMP-A.





